New Device Sparks Optimism in Fight Against Rheumatoid Arthritis
31.07.2025
New Vagus Nerve Implant Offers Fresh Hope for Rheumatoid Arthritis Sufferers
FDA Approves Innovative Device for Chronic Condition
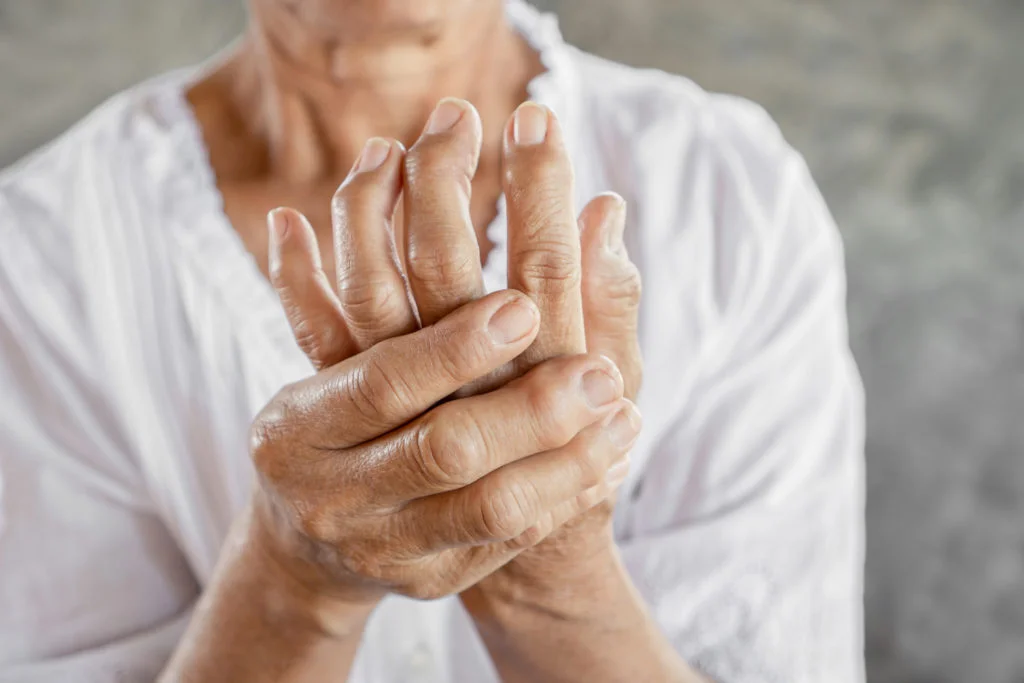
In a major step forward for the treatment of rheumatoid arthritis, the Food and Drug Administration has approved a novel medical device that may offer relief to the 1.5 million Americans living with the often disabling autoimmune disease. Unlike conventional drug-based treatments, the new implant harnesses the nervous system to reduce inflammation from within.
The device, known as the SetPoint System, is surgically implanted into the neck and wraps around the vagus nerve—the body’s longest cranial nerve. Once in place, it delivers a brief electrical stimulation each day to help regulate immune response and curb inflammation, which is a key driver of rheumatoid arthritis symptoms.
A Shift From Traditional Therapies
Most existing treatments for rheumatoid arthritis involve medications that suppress the immune system. While often effective, these drugs can leave patients vulnerable to serious infections and come with long-term side effects. The SetPoint System represents a dramatic departure from this approach. Instead of blocking immune pathways with chemicals, it aims to “retrain” the immune system by sending signals through the brain’s natural pathways.
The inch-long device was granted “breakthrough” status by the FDA last year, allowing for a faster review and approval process. Its development is part of the growing field of bioelectronic medicine, which explores the use of electrical signals to manage inflammation—a process central not only to rheumatoid arthritis but also to heart disease, diabetes, and certain cancers.
Promising Trial Results
In a randomized clinical trial involving 242 patients, more than half of those who received the SetPoint implant experienced significant improvement. Measures of joint pain and swelling fell by more than 60%, with some patients reaching full remission. Notably, these outcomes were achieved without suppressing the immune system.
The most commonly reported complication was mild hoarseness related to the surgical procedure, occurring in fewer than 2% of participants. While the FDA has approved the device, it has also mandated ongoing post-market surveillance to monitor for potential long-term risks, including infection—a common concern with implanted devices.
Patient Experience: A Life-Changing Device
Dawn Steiner, a 58-year-old speech pathologist from New York, participated in the clinical trial and said the implant dramatically improved her quality of life. After trying eight different biologic drugs with limited or temporary success, Steiner received the implant in mid-2023. Since then, she has reported significant mobility improvements and a substantial reduction in joint swelling and pain.
“Before the implant, my pain was a constant six or seven out of ten,” she shared. “Now I’m closer to a two.” She also noted that the device allowed her to enjoy social events again—something she had avoided due to the physical limitations imposed by her condition.
Perhaps most importantly, she is no longer immunocompromised, a risk she faced while taking standard RA medications.
A New Frontier in Autoimmune Care
The science behind the implant traces back to decades of research led by Dr. Kevin J. Tracey, a neurosurgeon and president of the Feinstein Institutes for Medical Research. He describes the vagus nerve as the body’s inflammation control center—comparable to a brake system in a car. If the nerve is functioning properly, the brain can “shut down” excess inflammation that contributes to chronic disease.
Dr. David Chernoff, chief medical officer at SetPoint Medical, said the goal isn’t to block immune response like traditional drugs, but to reset it. “We’re not disabling the immune system. We’re teaching it to respond appropriately.”
Risks, Costs, and Future Outlook
Although the exact price of the device hasn’t been released, the company stated that it’s built to last around 10 years and would be more cost-effective than some RA medications, which can cost thousands of dollars annually.
Medical experts have urged cautious optimism. Dr. Aaron Kesselheim of Harvard Medical School noted that fast-tracked FDA approvals often require post-market updates based on real-world usage. Meanwhile, Dr. Lou Bridges, a leading rheumatologist, welcomed the innovation but warned that long-term efficacy remains to be proven.
“This could be a groundbreaking approach,” Bridges said. “But we’ve seen bold claims before. Time and long-term data will ultimately tell the story.”
Looking Ahead
Clinical trials are already underway to explore similar vagus nerve stimulation treatments for other inflammatory conditions, including lupus, Crohn’s disease, and pediatric IBD. If successful, the SetPoint device could pave the way for a new era of drug-free, nerve-based therapies for autoimmune diseases.
For now, it stands as a beacon of hope for patients who have struggled to find relief through conventional treatment.