New Limits Set as FDA Grants Approval for Covid Shots
27.08.2025
F.D.A. Approves Covid Vaccines With New Restrictions
Limited Access Under New Policy
The Food and Drug Administration on Wednesday authorized updated Covid vaccines for the fall season but introduced strict eligibility limits, marking the most restrictive federal approach since the shots were first released. The vaccines were approved for adults 65 and older and for younger individuals with at least one underlying health condition. Children under 18 could still receive doses if cleared by a health provider.
Kennedy’s Influence on Vaccine Policy
The shift reflects Health Secretary Robert F. Kennedy Jr.’s longstanding skepticism of vaccines. He has restructured the CDC’s advisory panel, cutting its size and replacing members with several vocal vaccine critics. This advisory committee must still vote on whether to recommend the newly authorized shots, a step that will heavily influence distribution through pharmacies and state programs.
A Break From Tradition
Unlike in previous years, the FDA did not issue a press release on its approvals. This fall could be the first season in which Covid vaccines are not broadly recommended for the general population, setting up conflict between the Trump administration’s health officials and leading medical groups opposed to the restrictions.
Broader Campaign Against mRNA
Public health experts say the decision fits into Mr. Kennedy’s wider opposition to vaccines using mRNA technology. Earlier this year, he canceled $500 million in research grants for flu and Covid vaccines, a move critics say will stall innovation and leave the nation dependent on older methods.
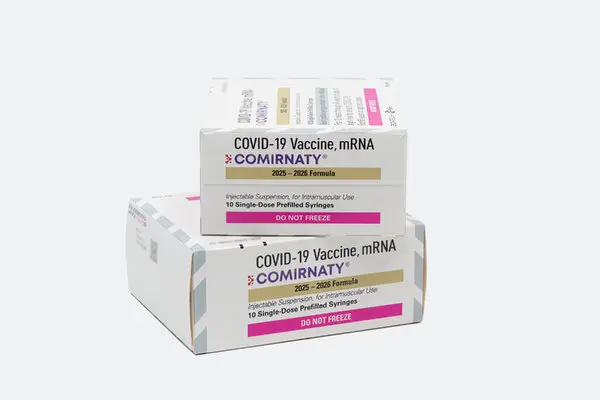
Details of the Authorization
Both Moderna and Pfizer received approvals for their updated mRNA vaccines, designed to target the Omicron variant LP.8.1. Moderna’s shot is cleared for people six months and older with medical conditions, as well as everyone over 65. Pfizer’s vaccine was authorized for similar groups beginning at age five.
Declining Vaccination Rates
Federal officials note that vaccine uptake has fallen in recent years: about 23 percent of adults and only 13 percent of minors received shots last year. Supporters of limiting eligibility argue that younger, healthy people face much lower risks of severe illness.
Pharmacies and Insurance Concerns
Pharmacists, who administer most Covid vaccines, are voicing concerns about legal uncertainty tied to the restrictions. Some states only allow pharmacy staff to provide vaccines endorsed by the CDC. Insurance companies have indicated they will continue coverage, though questions remain about Medicaid and private plans tied to the new recommendations.
Opposition From Medical Groups
Several national medical organizations are pushing back against Kennedy’s changes. In May, the CDC stopped recommending vaccines for pregnant women. The American College of Obstetricians and Gynecologists (ACOG), however, continues to advise vaccination during pregnancy, citing strong evidence that shots reduce maternal and infant risks. Doctors warn that restrictions could increase preventable deaths.
Legal and Political Fallout
Six leading medical groups have sued the Department of Health and Human Services, arguing that the new rules could cost lives, including those of pregnant women and newborns. Meanwhile, states such as Massachusetts, Maryland, and Pennsylvania are exploring ways to maintain access to vaccines regardless of federal policy shifts.
Current Covid Trends
Although overall Covid deaths have dropped sharply, cases have ticked upward in recent weeks, particularly among children. Emergency rooms across the country are reporting more young patients, though most are not severely ill unless they have other health complications.
Looking Ahead
Traditionally, the FDA grants broad approvals while the CDC decides on recommendations for specific groups. This year’s approach — limiting access at the FDA level — represents a major departure. With Kennedy’s direct involvement in CDC decisions, the battle over vaccine policy is likely to intensify as states, medical groups, and insurers weigh how to respond.